| Accuracy | |
| Valid for practice | |
| True to literature | |
| Overall quality |
Please peer-review this blog post by clicking on the stars above.
The story of vancomycin all started when a missionary from Boreno sent a sample of dirt to a friend at Eli Lilly. The compound isolated had activity against most gram positive organisms. In fact, it got its name from the word 'vanquish.' Vancomycin was FDA-approved in 1958. [1]
Vancomycin is still a powerful tool against gram positive organisms, but there are some important learning points for using it properly in the critically ill ED patient.
First, a little history...
The original minimum inhibitory concentration (MIC) cutoff for S. aureus susceptibility was ≤ 4 mcg/mL. This was decreased to ≤ 2 mcg/mL in 2007 despite the emergence of vancomycin intermediate S. aureus (VISA). [2] Remember, MIC is the lowest concentration of an antimicrobial that will inhibit the visible growth of a microorganism after overnight incubation (thanks Wikipedia). MICs can vary based on testing method utilized (by 1-2 fold).
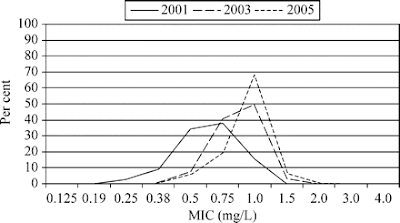
What is MIC Creep?
Over time it seems the MIC needed for vancomycin to eradicate MRSA is increasing, as evidenced by increasing vancomycin MIC distribution in S. aureus isolates. [3]
What's the problem?
Patients are dying from bacteremias even with MICs ≤ 2 mcg/mL that appear as 'susceptible' on institutional reporting tools. This is scary!
- Vancomycin MIC of 2 mcg/mL was an independent predictor of mortality. [4]
- MIC break point ≥ 1.5 mcg/mL was associated with an increased probability of vancomycin treatment failure. [5]
These two findings were verified by a systematic review/meta-analysis. [6] The authors also found that vancomycin treatment failures and mortality for MRSA infections occurred regardless of source of infection or MIC testing methodology!
What it all means
Although in the ED we often don't have access to the patient's susceptibility data, make sure to look at previous records from your institution or the transferring institution. Just because the culture report says 'S' (for susceptible), the MIC may be between 1.5 and 2 mcg/mL.
- For bacteremias/endocarditis: if the S. aureus MIC ≥ 1.5 mcg/mL, don't use vancomycin!
- For all other MRSA infections: if the S. aureus MIC ≥ 2 mcg/mL, don't use vancomycin!
Proper Vancomycin Dosing in the ED [7]
- Stop using a default dose of 1 gm for all patients. Vancomycin is dosed based on total body weight.
- An initial dose of 15-20 mg/kg should be started in the ED. For critically ill patients, loading doses (25-30 mg/kg) can be considered.
- The initial dose should not be adjusted down based on renal function (we can adjust the dosing interval later).
Thanks to Emily Heil, PharmD, BCPS for providing much of the information for this post.
Original: January 16, 2013
Last updated: January 17, 2013
References
- Levine DP. Vancomycin: A History. Clin Infect Dis 2006;42(Supplement 1):S5-S12.
- Performance Standards for Antimicrobial Susceptibility Testing; Twenty-Second Informational Supplement. CLSI Document M100-S22. Wayne, PA: Clinical and Laboratory Standards Institute; 2012.
- Steinkraus G, et al. Vancomycin MIC creep in non-vancomycin-intermediate Staphylococcus aureus (VISA), vancomycin-susceptible clinical methicillin-resistant S. aureus (MRSA) blood isolates from 2001-05. J Antimicrob Chemother 2007;60(4):788-94. [PMID 17623693]
- Soriano A, et al. Influence of vancomycin minimum inhibitory concentration on the treatment of methicillin-resistant Staphylococcus aureus bacteremia. Clin Infect Dis 2008;46(2):193-200. [PMID 18171250]
- Lodise TP, et al. Relationship between vancomycin MIC and failure among patients with methicillin-resistant Staphylococcus aureus bacteremia treated with vancomycin. Antimicrob Agents Chemother 2008;52(9):3315-20. [PMID 18591266]
- van Hal SJ, et al. The clinical significance of vancomycin minimum inhibitory concentration in Staphylococcus aureus infections: a systematic review and meta-analysis. Clin Infect Dis 2012;54(6):755-71. [PMID 22302374]
- Rybak MJ, et al. Therapeutic monitoring of vancomycin in adult patients: a consensus review of the American Society of Health-System Pharmacists, the Infectious Diseases Society of America, and the Society of Infectious Diseases Pharmacists. Am J Health Syst Pharm 2009;66(1):82-98. [PMID 19106348]